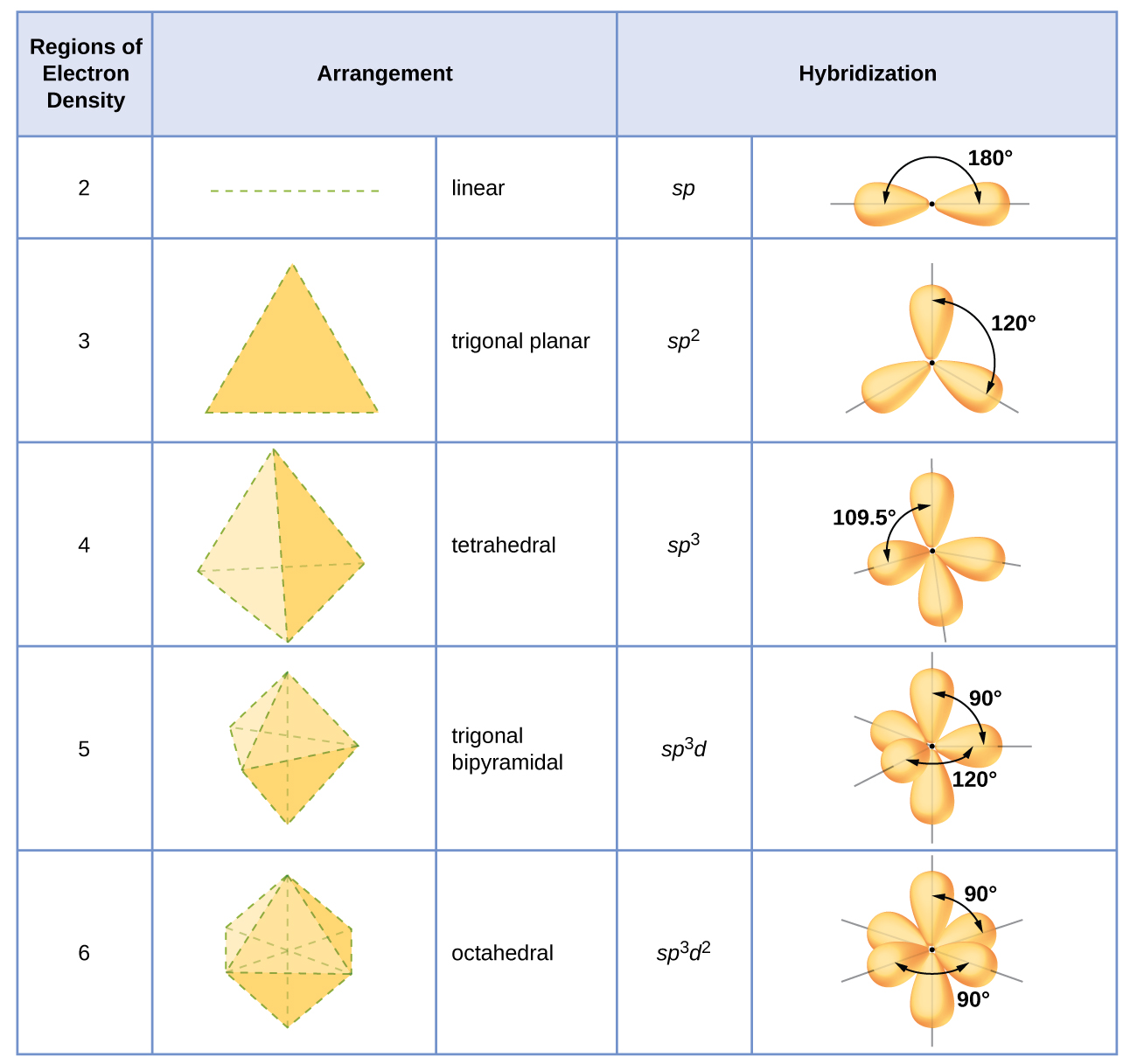
Describe the Hybrid Orbitals Used by the Central Atom
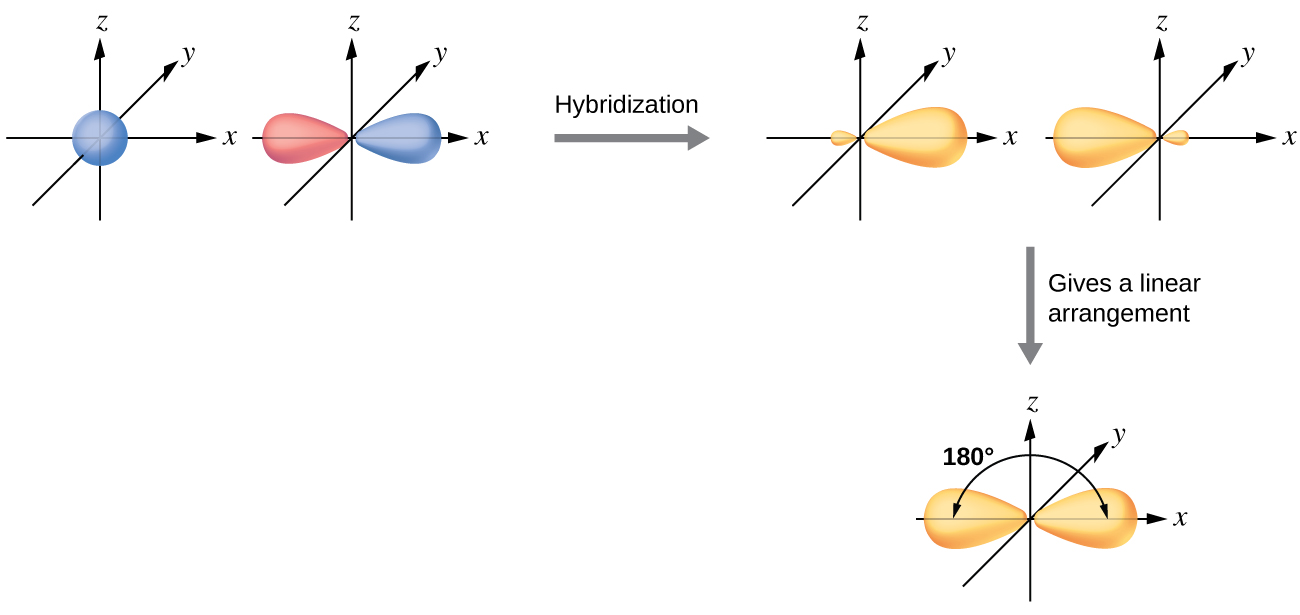
Valence orbitals of the atoms mix to become new hybrid orbitals in the molecule. Hybridization can also be calculated for a molecule using the formula.
Solved Describe The Hybrid Orbitals Used By The Central Atom And The Type S Of Bonds Formed In A Mathrm No 3 B Mathrm Cs 2 Mathrm C Mathrm Ch 2 Mathrm O
There is no lone electron pair on Si atom.
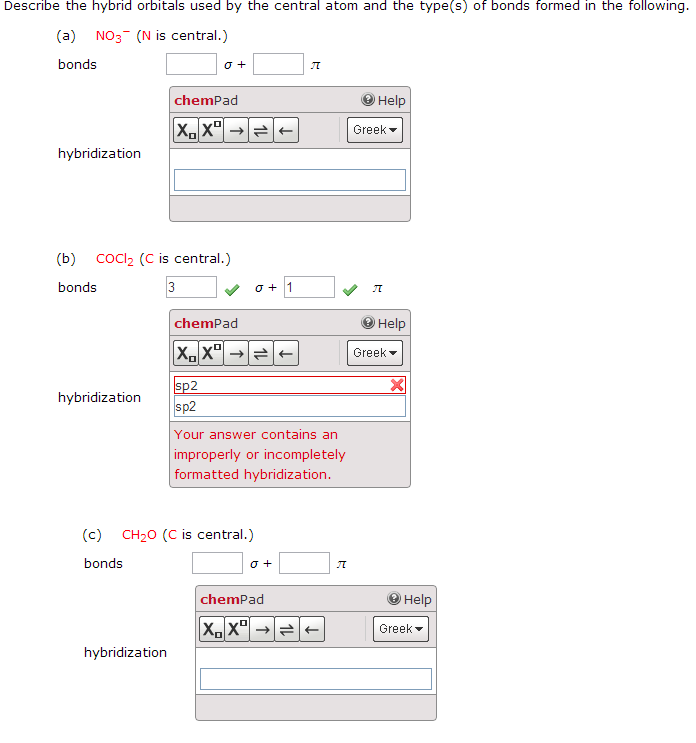
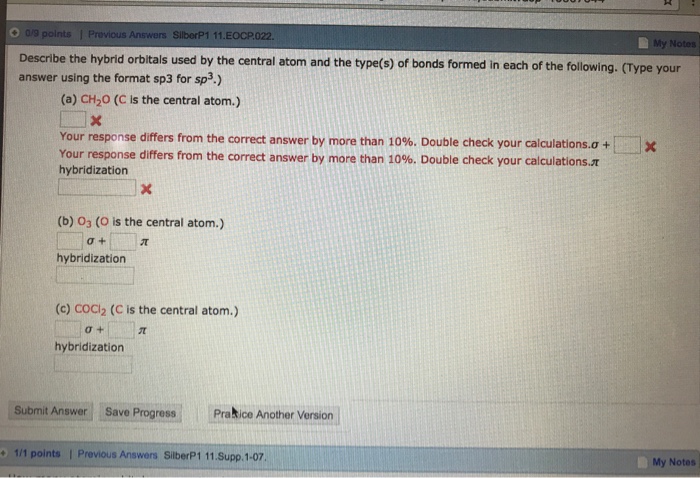
. For formaldehyde X ½ 2 4 0 0 3. Fill these hybrid orbitals with the total number of valence electrons around the central atom and describe the hybridization. The carbon is bonded to two other atoms that means it needs two hybrid orbitals aka sp.
The spatial orientations of hybrid orbitals match the observed molecular shapes. In the above structure the central bromine atom is surrounded by 5 electron groups three single bonds and two lone pair electrons. Just like the carbon atom in methane the central nitrogen in ammonia is sp 3-hybridized.
You describe the molecular geometry around each central atom separately. Find step-by-step Chemistry solutions and your answer to the following textbook question. Describe the hybrid orbitals used by the central atoms and the types of.
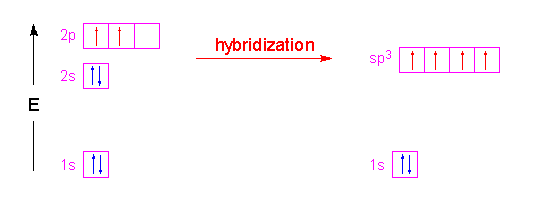
The Valence Bond Theory is the first of two theories that is used to describe how atoms form bonds in molecules. Each C-H bond in methane then can be described as a sigma bond formed by overlap between a half-filled 1s orbital in a hydrogen atom and the larger lobe of one of the four half-filled sp 3 hybrid orbitals in the central carbon. 1 bond to another atom or lone.
Valence bond theory proposes that before a covalent bond forms atomic orbitals from a given atom can combine to form new atomic orbitals. Every lone pair needs it own hybrid orbital. Hybridization c CS2 C is the central atom.
Which one of the following molecules is polar. C cationic charge. Give the number and type of hybrid orbital.
X ½ H V A C where. That makes three hybrid orbitals for lone pairs and the oxygen is bonded to one hydrogen which requires another sp 3 orbital. Describe the hybrid orbitals used by the central atoms and the types of.
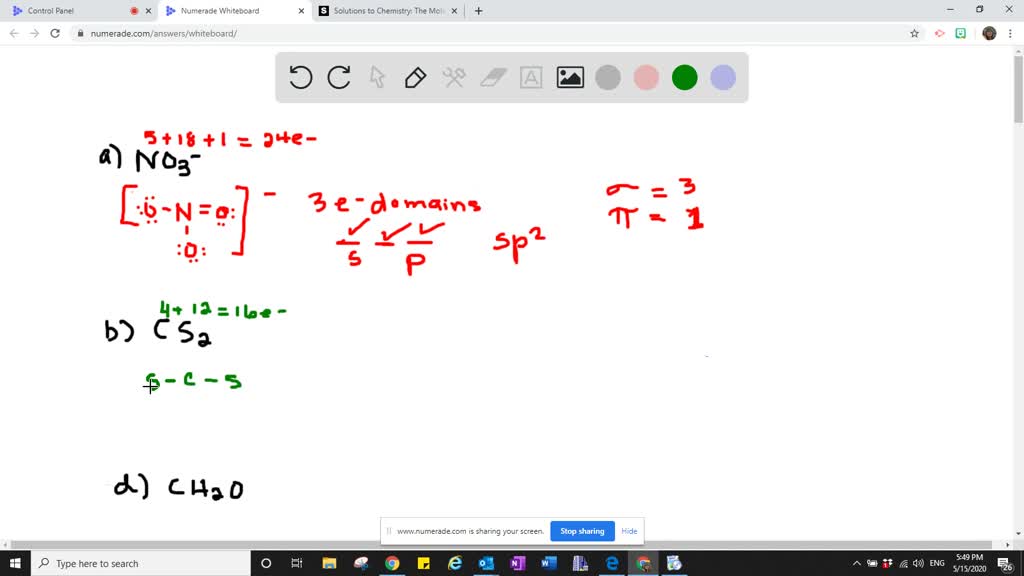
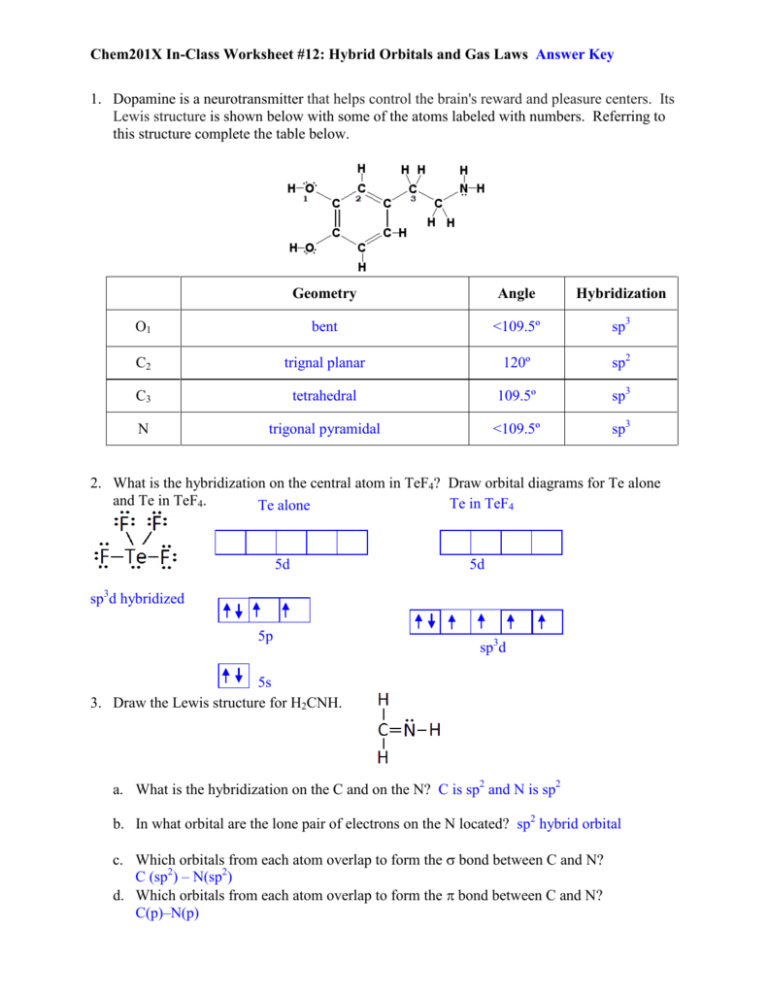
Type your answer using the format sp3 for sp3 a CH2O C is the central atom. Describe the hybrid orbitals used by the central atom and the types of bonds formed in a NO3-. So the steric number of Si atom is 4.
Therefore five hybrid orbitals are required for hybridization. Indicate the type of hybrid orbitals used by the central atom in CCl4. The central atom Si is attached to four Cl atoms through four sigma bonds.
Describe the hybrid orbitals used by the central atom and the types of bon 0705 Describe the hybrid orbitals used by the central atoms and the types of. That makes 4 orbitals aka sp 3. A anionic charge.
With nitrogen however there are five rather than four valence electrons to account for meaning that three of the four hybrid orbitals are half-filled and available for bonding while the fourth is fully occupied by a non-bonding pair of electrons. 0017 A molecule in which sp 2 hybrid orbitals are used by the central atom i. In which of the following compounds will the molecules not form hydrogen bonds with each other.
Describe the hybrid orbitals used by the central atom and the types of bonds formed in each of the following. Describe the hybrid orbital set used by each of the indicated atoms in the m 0729 Describe the hybrid orbitals used by the central atom and the types of bon. The length of the carbon-hydrogen bonds in methane is 109 pm.
C1 sp2 - C2 sp3. So one 3s three 3p and one d orbitals mix to form five dsp 3 hybridized orbitals. Solution for Describe the hybrid orbitals used by the central atom and the types of bonds formed in a NO₃.
The notion of hybrid orbitals was invented by Linus Pauling in the 1930s as a way. Of monovalent terminal atoms. A H 2 S has four electron pairs around the sulfur atom with two bonded atoms so the VSEPR model predicts a molecular geometry that is bent or V shaped.
Identify the compound with atoms that have an incomplete octet. 0706 Which types of atomic orbitals of the central atom mix to form hybrid orbita. Determine the electron and molecular geometries give the hybridization of the central atom and determine if the molecule is polar or nonpolar.
Of valence electrons. Describe the hybrid orbitals used in forming the bond between C-1 and C-2. Configuration and then all four of the orbitals become hybridized to a uniform energy level as 1s 3p 4 sp 3 hybrid orbitals.
The following rules give the hybridization of the central atom. So the hybridization of Si in SiCl4 is sp3. Hence to form 4 sigma bonds four hybridized orbitals are required.
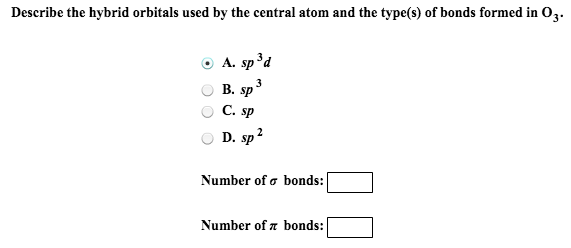
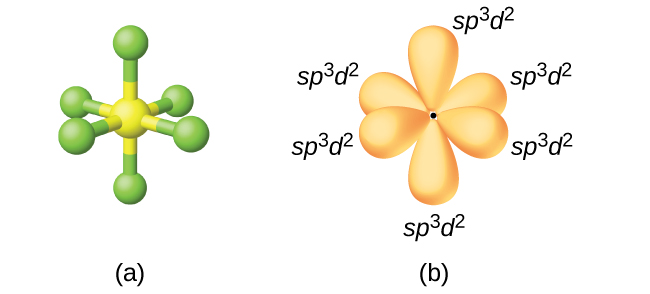
Hence the hybridization bromine in molecule is dsp 3. The central atom exhibits sp2 hybridization since there is trigonal planar electron pair geometry. Of valence electrons in the central atom.
8 2 Hybrid Atomic Orbitals Chemistry
Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com
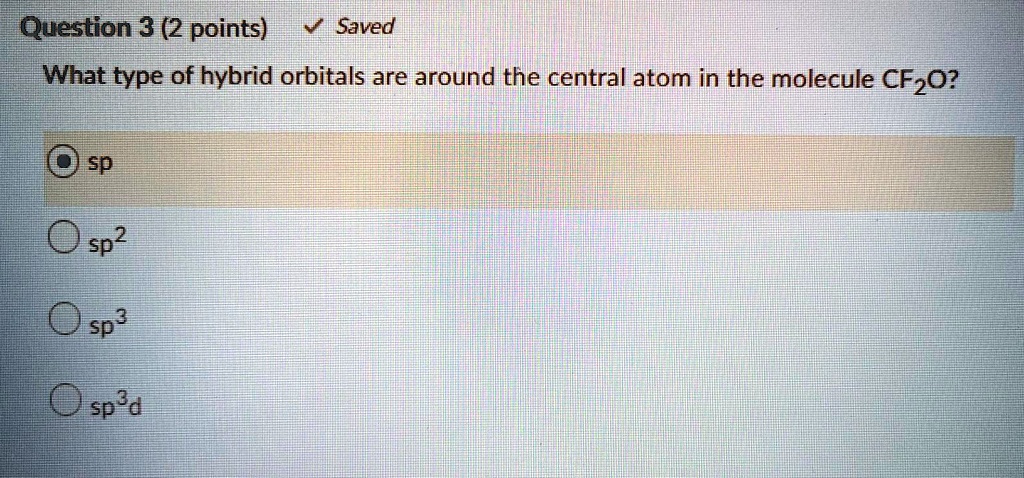
Solved Question 3 2 Points Saved What Type Of Hybrid Orbitals Are Around The Central Atom In The Molecule Cf2o Osp O Sp2 0 Sp 0 Sp D
Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com

Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com
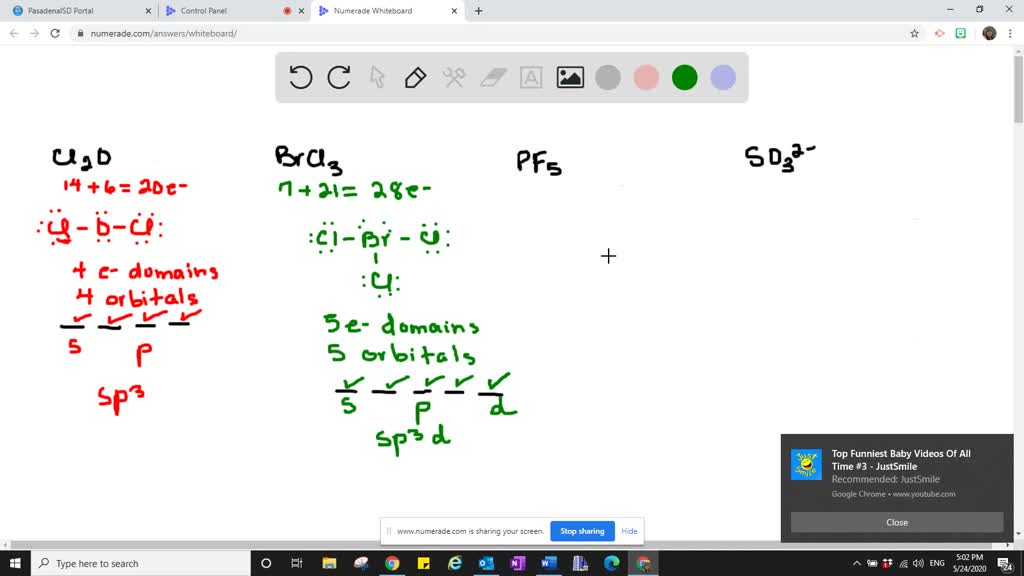
Solved Which Types Of Atomic Orbitals Of The Central Atom Mix To Form Hybrid Orbitals In A Mathrm Cl 2 Mathrm O Mathrm B Mathrm Brcl 3 Mathrm C Mathrm Pf 5 Mathrm D Mathrm So 3 2
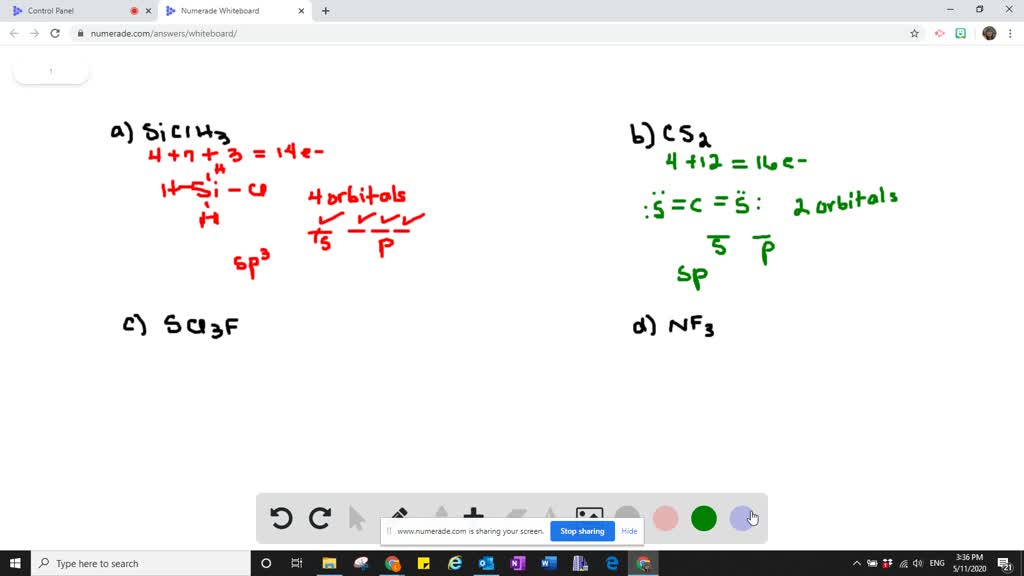
Solved Which Types Of Atomic Orbitals Of The Central Atom Mix To Form Hybrid Orbitals In A Mathrm Siclh 3 B Mathrm Cs 2 C Mathrm Scl 3 Mathrm F D Mathrm Nf 3
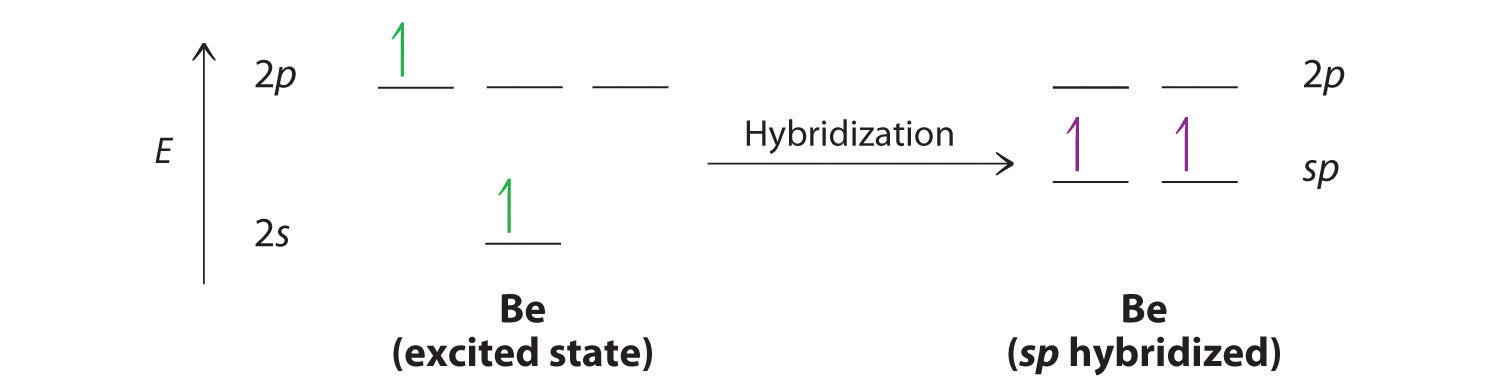
10 2 Hybrid Atomic Orbitals Chemistry Libretexts
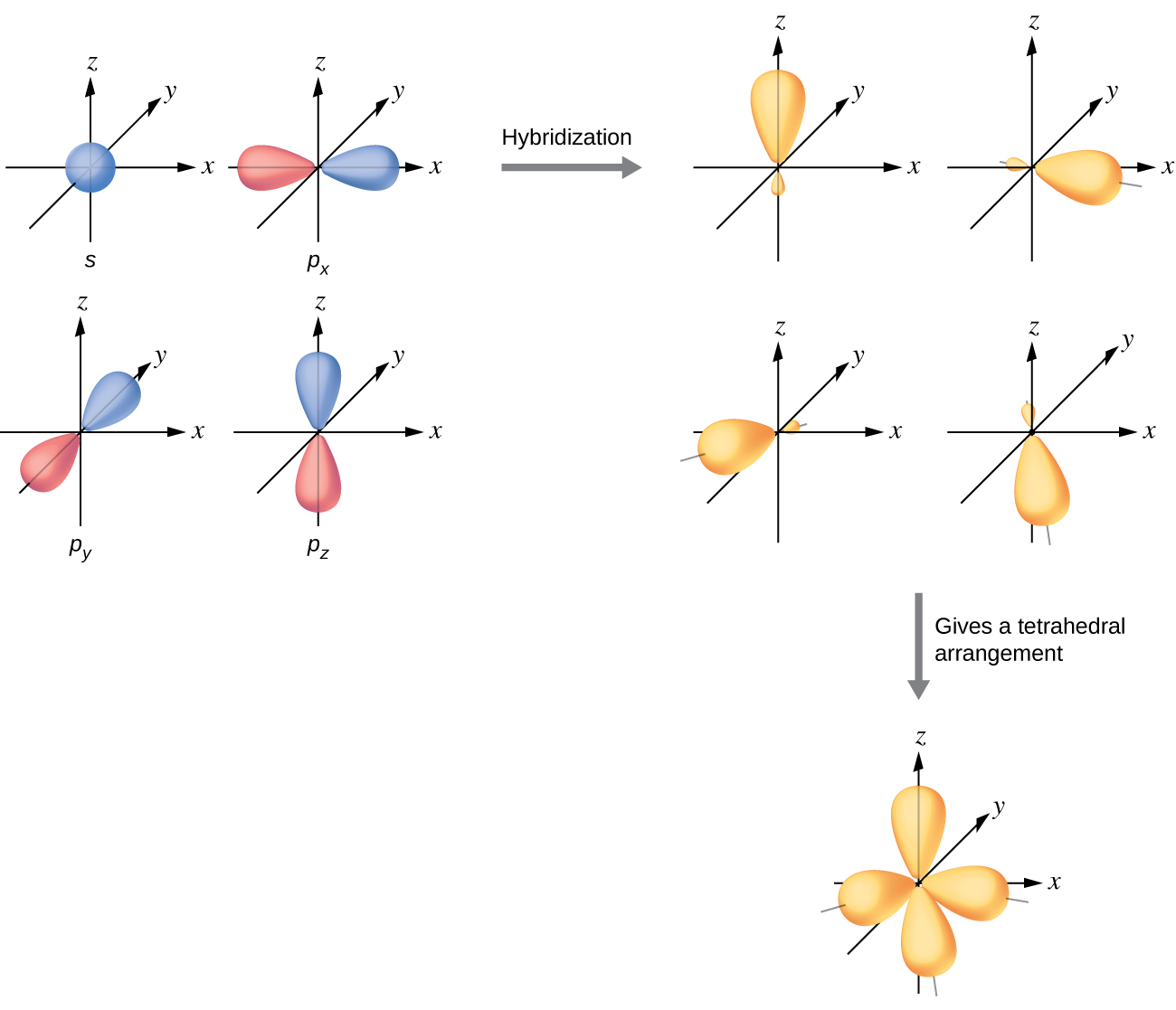
8 2 Hybrid Atomic Orbitals Chemistry

Question Video Identifying The Number Of Hybrid Orbitals Formed In Sp 𝑛 Hybridization Nagwa
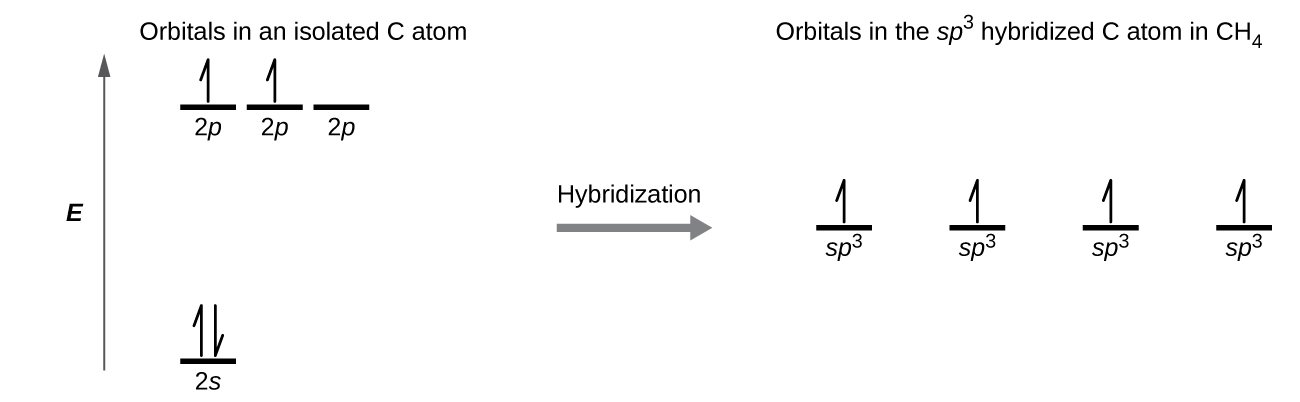
8 2 Hybrid Atomic Orbitals Chemistry
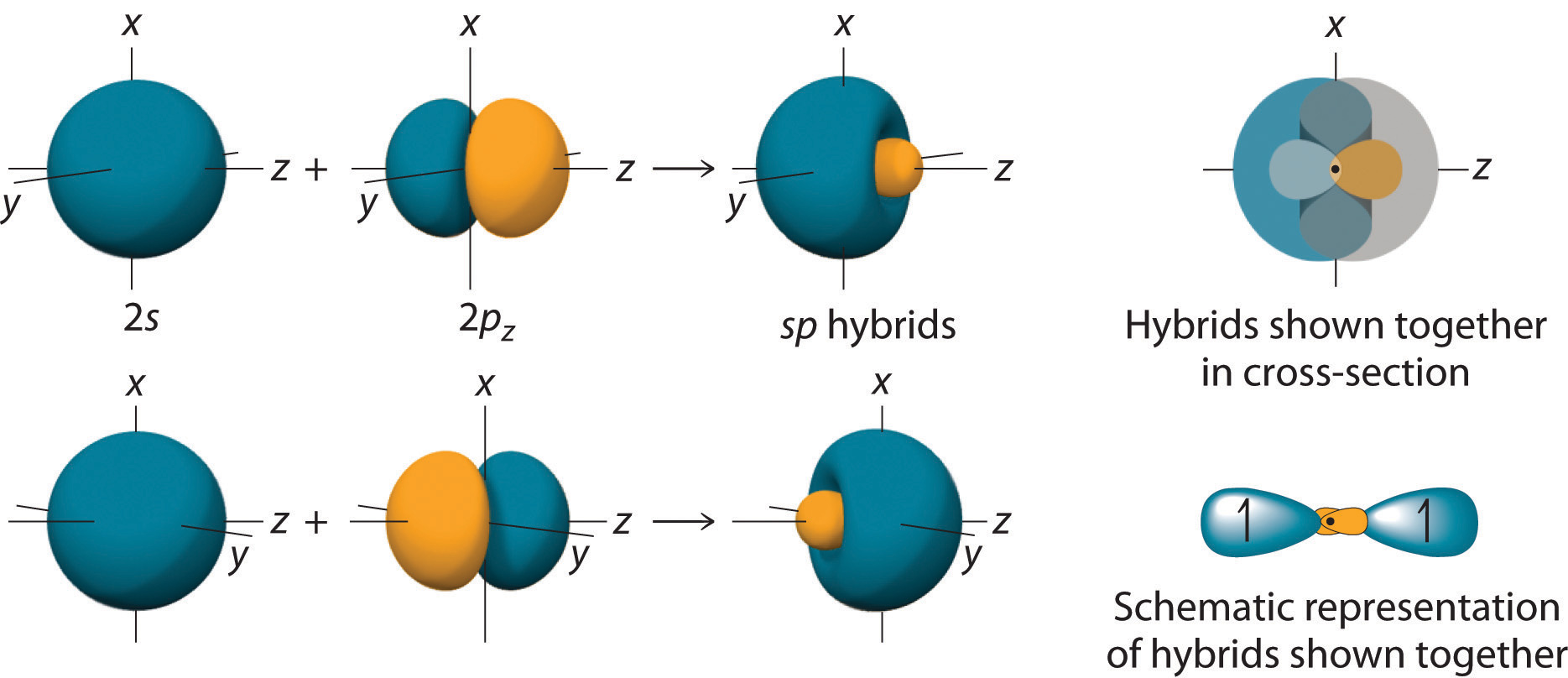
10 5 Hybrid Orbitals Chemistry Libretexts
8 2 Hybrid Atomic Orbitals Chemistry
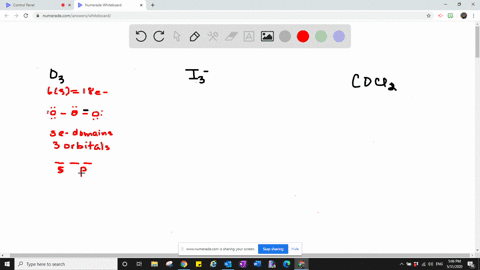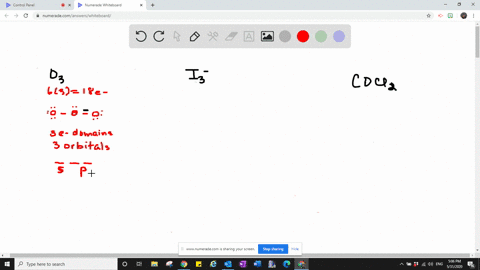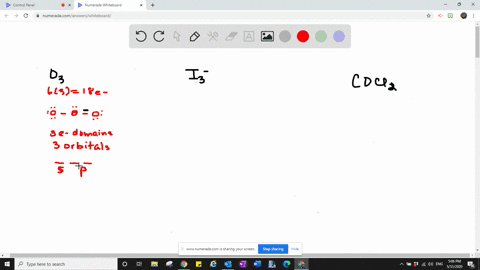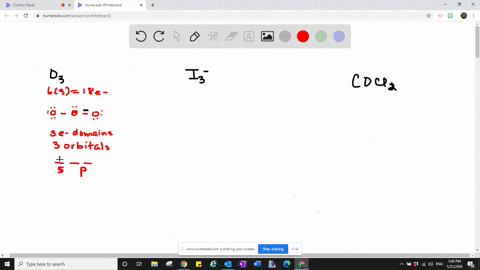
Solved Describe The Hybrid Orbitals Used By The Central Atom And The Type S Of Bonds Formed In A Mathrm No 3 B Mathrm Cs 2 Mathrm C Mathrm Ch 2 Mathrm O

Solved Describe The Hybrid Orbitals Used By The Central Atom Chegg Com

Hybridization In Molecules Containing Double Triple Bonds Video Lesson Transcript Study Com
Comments
Post a Comment